This report is dedicated to each and every child diagnosed with a brain tumor


CBTN Vision
By accelerating the pace of translational research and the discovery of new treatments, we are a global community with the shared goal to save children and young adults from brain tumors.
CBTN Mission
The Children’s Brain Tumor Network is dedicated to driving innovative discovery, pioneering new treatments and accelerating open science to improve health for all children & young adults diagnosed with a brain tumor
Michelle Monje, MD, PhD, (on right) Principal Investigator at Lucile Packard Children’s Hospital Stanford and member of the CBTN’s Executive Board and Scientific Committee, together with members of the Monje Laboratory team at Stanford. Photo courtesy of Lucile Packard Children’s Hospital Stanford.
Adapting to the COVID-19 Pandemic
A NEW RESEARCH CHALLENGE
The global shutdown resulting from the COVID-19 pandemic has significantly impacted our CBTN research network. Laboratory research at numerous member sites was put on hold until new safety protocols could be implemented. Collection, analysis, and sharing of tumor tissue and other biospecimens also paused. Our partner foundations and patient families especially felt the effects of the pandemic on their fundraising and advocacy efforts, but found creative new ways to continue to support the CBTN’s research.
As this public health crisis continues to impact communities around the world, the CBTN’s commitment remains undeterred. Thanks to the CBTN’s suite of cloud-based analysis platforms, data is accessible from anywhere on earth, allowing research projects to move forward. As new health and lab safety measures are implemented, biospecimen collection and analysis are steadily returning to their pre-pandemic levels. CBTN’s patient advocates and research supporters at every level are answering the call to address the unexpected funding challenges. Thanks to these generous financial gifts, the CBTN has continued the development of global collaborations, increased the number of samples in the biorepository, and shared vital data to enable advancements in innovative brain cancer research.
The current pandemic is demonstrating the critical role that global collaboration is playing in the development of treatments for COVID-19. Working together has enabled the medical community to make discoveries faster than ever before. The CBTN was applying these lessons to the treatment of childhood brain tumors, even before the pandemic began. Collaboration and global partnership are woven in the very fabric of the CBTN.
The CBTN is committed to treating childhood brain tumors with the same level of urgency and collaboration as was seen during the development of the COVID-19 vaccines. No single institution can collect enough specimens or data in isolation to make significant advances in brain tumor treatment, so it’s critical to leverage every available resource on behalf of affected children.
The success of the CBTN is thanks to the committed teams of clinicians, researchers, patient families, and many others who won’t stop until every child is cured. The CBTN is extremely grateful for the dedication and partnership of each of these collaborators. Together, we’ve made tremendous strides in our understanding of brain tumor biology and will continue to work to accelerate treatments for each and every patient.
Adapting to a new era of research
- Globally, the COVID-19 pandemic touched nearly all facets of society, including clinical operations, research, and philanthropy across the CBTN.
- Throughout the pandemic, CTBN and its partners identified additional needs of the childhood brain tumor community.
- Each of the CBTN’s investigators, clinicians, and research coordinators adapted to new research challenges, and by the end of Fiscal Year 20, resumed our tissue collection and analysis efforts across the network.
- The CBTN’s cloud-based analysis platforms enabled the CBTN to increase and expand the amount of research data available to researchers across the globe.
Leadership Perspective

The leadership team of the Children’s Brain Tumor Network (CBTN) could not reflect on this transformative year without first highlighting CBTN’s expanded mission and rebranding from our roots as the Children’s Brain Tumor Tissue Consortium (CBTTC).
This evolution from CBTTC to CBTN ultimately represents our re-commitment to the same values that launched this partnership in 2011 — that innovation and collaboration, combined with a shared vision, will create a future where no child suffers or dies from a brain tumor. The growth we’ve experienced during an unprecedented 2020 has not come without challenges, but the CBTN’s unwaiver- ing commitement to think differently and innovate have defined a successful path forward for years to come.
The events of this past year have revealed areas of additional need, including disparities in academic research and clinical care. Investigators from CBTN and the Pacific Pediatric Neuro-Oncology Consortium (PNOC), led by Drs. Cassie Kline and Angela Waanders, are working to address injustice and biases within the brain tumor community, to improve access to care for all patients.
Creative solutions have led to significant triumphs by the CBTN during Fiscal Year (FY) 20. Nineteen research papers utilizing CBTN’s data and specimens were published in 13 scientific journals, including a landmark study published in partnership with the National Cancer Institute’s Clinical Proteomic Tumor Analysis Consortium (CPTAC). This collaborative effort produced a first-in-kind, large-scale analysis of the “proteogenomics” or the proteins of pediatric tumors.
Among several new initiatives has been the establishment of joint CBTN & PNOC disease working groups, each consisting of experts across a number of pediatric brain tumor diagnoses and research emphases. The new working groups each include representation from the clinical, research and patient communities. New working groups are also being considered for many of the especially uncommon types of childhood brain tumors, with the goal to identify and develop new precision clinical trials and therapeutic options for children with brain tumors.
The effects of the global pandemic have significantly impacted CBTN’s foundation and philanthropic partnerships. Through the dedication of CBTN’s foundation supporters and families, we’ve been able to sustain and even increase our research activities on behalf of all children with brain tumors. Our network of supporters has responded with new ways to provide the necessary funding for CBTN’s operations and expansion.
Thanks to a generous $1.75 million investment from the Swifty Foundation early in FY 20, 13 new personnel have been added within CBTN’s Research Core and five research initiatives designed for CBTN have been launched to achieve a new level of operational excellence. This increase in experts on staff has thus far enabled the creation of diagnostic workflows critical to the support of clinical trials through our disease working groups.
These infrastructure improvements have better positioned CBTN to secure sustainable federal funding from the National Institutes of Health (NIH). In FY20, CBTN submitted its first-ever pediatric brain tumor SPORE (Specialized Programs of Research Excellence) application to the National Institutes of Health, which represented a key opportunity to secure ongoing federal research funding. Results of Pediatric Brain Tumor SPORE submission are expected in FY21.
Global opportunities, unprecedented challenges, and significant achievements have abounded this year, and we, the leadership of the Children’s Brain Tumor Network, have been humbled and gratified to work in service of CBTN’s mission for scientific discoveries that will benefit today’s children
Gratefully,


A new face on the leadership team this year is Dr. Brian Rood of Children’s National Hospital, who became Co-Chair of the CBTN Executive Board this summer.
A CBTN investigator since 2015, Dr. Rood brings with him a drive to support collaborative science, transparent data sharing, and the translation of scientific discoveries into new treatments for children and young adults suffering from brain tumors around the world.
Photo courtesy of Children’s National Hospital
From CBTTC to CBTN
A NEW IDENTITY
The change in our organization’s identity from the Children’s Brain Tumor Tissue Consortium to the Children’s Brain Tumor Network marks a new phase in our efforts on behalf of childhood brain tumor patients everywhere.
Now as the CBTN, we will further grow our partnerships and research efforts to cure pediatric brain tumors through innovation, resource sharing, a commitment to global inclusion, and most critically, the translation of data into new clinical trials and increased clinical impact. Through this expanded mission, we are determined to uncover more effective and less toxic therapies to benefit TODAY’s children.

Measuring Our Growth
AN EXPANDED MISSION

In order to further accelerate research progress, CBTN is partnering with foundations, public agencies, commercial/pharmaceutical partners, and academic institutions to vastly expand the number of biospecimen and data research projects, as well as supporting increases of precision medicine trials while utilizing CBTN resources to inform trial development and deployment through our partners at PNOC.
Despite the effect of COVID-19 on research across the world, the CBTN continued to expand. A total of 3,546 subjects have been enrolled (an increase of 202 this fiscal year), 45,686 aliquots of precious samples have been added to the biorepository, and highly-valuable, longitudinal clinical data, reports and imaging were collected to enhance the genomic data and samples available.
CBTN not only grew in samples and data, but the network expanded in strategic partnerships with the addition of three new member institutions. Hassenfeld Children’s Hospital at NYU Langone (New York, New York) joined as a primary member site, and both Orlando Health Arnold Palmer Hospital for Children (Orlando, Florida) and Michigan Medicine C.S. Mott Children’s Hospital (Ann Arbor, Michigan) joined as satellite members. At the time of this report, CBTN has approved seven new member sites, all of which are currently onboarding.
In another example of our drive to find cures for our children, CBTN celebrated a significant milestone during Brain Tumor Awareness Month in May. The 100th data project was approved by the CBTN Scientific Committee, exemplifying our commitment to open science and open access to our resources.
Patient Partnerships

Foundation Partnerships
A NEW FOUNDATION COUNCIL

During FY20, CBTN worked to better understand and support the needs of the patient community by hosting listening tours with each of CBTN’s
Advisory Council member foundations. The Advisory Council, launched in 2017, was created with the goal of supporting CBTN’s vision and mission through advocacy, philanthropic and community-focused efforts.
Thanks to feedback and insights collected during the foundation listening tours, CBTN restructured its foundation and donor partnerships into a new Executive Council.
The new Executive Council will be charged with informing and partnering with CBTN leadership, acting as community stakeholder representatives, and driving the national and global impact of CBTN.
Executive Council members provide a minimum annual gift of $25,000 and participate in quarterly meetings over a two-year membership commitment. Executive Council members also support CBTN through their service in one of two subcommittees, the Finance & Funding Subcommittee or the Advocacy & Awareness Subcommittee.
If you are interested in learning more about the CBTN Executive Council, contact Gerri Trooskin, Director of Partnerships at troosking@chop.edu
ADVOCACY IN ACTION: DIPG AWARENESS DAY
On Thursday, February 13, 2020, at the Rayburn House Office Building in Washington, DC, diffuse intrinsic pontine glioma (DIPG) patient and family advocates joined national experts in childhood brain tumor research, as well as representatives from government and industry, at a Congressional Briefing titled DIPG, Pediatric Brain Cancer and the Importance of H. Res. 114. Presented by the DIPG Advocacy Group in collaboration with Congresswoman Jackie Speier (D-CA), the event was held to urge the 116th Congress to pass U.S. House Resolution 114 (H. Res. 114).
H. Res. 114 calls for the designation of the 17th of May each year as “DIPG Awareness Day” to raise awareness and encourage research into cures for diffuse midline gliomas (including DIPG), and for childhood cancers in general; as well as to open opportunities for data-sharing, and to push for increased private and public funding for research and new clinical trials for treatment.
Additionally, the resolution would help to provide patient families and doctors with more information about available experimental research, clinical trials, and/or treatment strategies. beginning at the time of diagnosis.
CBTN and PNOC investigators Dr. Adam Resnick and Dr. Sabine Mueller joined a panel of experts to deliver remarks on the state of research for these conditions. Jace Ward, a 21-year-old college student who was diagnosed with DIPG brain cancer in May of 2019, also shared remarks about his brain tumor journey. Since his diagnosis, Jace has become a leading patient voice, speaking to researchers, funders, public policymakers, and the community at-large to instill a sense of urgency in the discovery of new treatment strategies.

DIPG is not rare. It’s just rarely talked about. As a nation, we are not aware. We are failing these patients and their families. Awareness will lead to more cures.
DIPG patient and research advocates display signs and photos of loved ones who passed away or were otherwise affected by a childhood brain tumor during a meeting at the Rayburn House Office Building in Washington D.C. (Photo courtesy of CBTN)
A CALL TO ACTION

Jace Ward is a junior at Kansas State University studying business and pre-law. On May 17, 2019, following multiple rounds of tests and uncertainty, Jace was diagnosed with a diffuse intrinsic pontine glioma, or DIPG. Jace has become a strong advocate for the brain tumor patient community by bringing attention to these under-represented conditions. His mantra throughout his treatment has been, “I can’t die, I’m busy.”
I have great hope and also frustration as I survey the landscape of data, research, and clinical trials. I feel the momentum rising in pockets like CBTN and yet I face the reality that my tumor won’t wait until we all agree on the most perfect ways to structure collaboration and trials.
Since my diagnosis 18 months ago, I have watched a child die nearly every single day from DIPG alone; with parents sharing their heartbreaking progression. In the last decade, 1.3 million years of life have been lost to pediatric cancer - each one stolen away from children with unique talents and purpose in the world.
What is to blame for the lack of progress in childhood brain tumor research? Is it lack of funding? Extreme caution in trials? Inability to effectively share and analyze data and biospecimens?
Whatever the reason, I can guarantee every patient family knows, without a doubt, how much more important it is to efficiently share their child’s data, tumor tissue, and biospecimens than to keep those valuable resources locked away. Each one would opt for broadly sharing these resources for one main reason: No family who has experienced the pain of childhood cancer would wish it on any other.
I have great hope because I have had some success in treatment when I have used my data through CBTN for analysis by trial investigators, pharmaceutical companies and doctors.
Still, far too few discoveries have been made in past decades and now we must play catch up. No one can better teach us bravery in action than a child with brain cancer. Let’s start right now by really seeing the child, each child, as if he or she is our own. Let’s work urgently to save them.
In the words of the late Congressman John Lewis, “If not us, then who? If not now, then when?”
Friends, we each get one life to make an impact. My case is not any different than yours. None of us know how many days we have on earth to do our best work. Use every single one. - Jace
“DIPG won’t wait. We know it is possible to use urgency to chart a new course; we have seen this during the COVID-19 crisis. Urgency requires a united commitment to somewhat rebellious collaboration. It requires deciding a patient deserves the right to life and the pursuit of lifeextending treatments. It will require bravery to change institutional thinking.”
Scientific Community Partnerships

Driving Discovery through Collaboration
PNOC 008
CBTN continued its collaborative efforts with the Pacific Pediatric Neuro-Oncology Consortium (PNOC) through the PNOC008 trial titled, ‘A Pilot Trial Testing the Clinical Benefit of Using Molecular Profiling to Determine an Individualized Treatment Plan in Children and Young Adults with High-grade Glioma’.
The first subject was enrolled in April of 2019. With a target of 1-2 subjects per month across any PNOC site that is IRB approved for the study, tumor samples are collected for clinical genomics, which are generated in the CLIA-certified lab at UCSF followed by the production of a clinical report.
If additional tissue is available, the sample is sent for research sequencing first via the Division of Genomic Diagnostics at CHOP for extractions and then to a third-party vendor to generate the data.
Once the data is received, analysis is run against the Pediatric Brain Tumor Atlas (PBTA) with another clinical report being produced. Both reports are provided to the PNOC Tumor Board for review and to aid in treatment decisions for the subject. The data then is incorporated back into the PBTA to inform on treatment decisions for future subjects. In 2020, 19 patients were enrolled, nine of which had sequencing completed through the division of Genomic Diagnostics at CHOP.
CBTN/PNOC WORKING GROUPS
This year, CBTN launched a new collaborative effort with our partners at the Pacific Pediatric Neuro-Oncology Consortium (PNOC), supporting our increased focus on clinical trial and clinical decision support. Seven CBTN/ PNOC Disease Working Groups have been formed to focus research efforts for a number of histologies and research areas.
Working groups have been established for: medulloblastoma, diffuse midline glioma, AT/RT, low-grade glioma, ependymoma, craniopharyngioma, and imaging. Each working group has their own goals, convene once every month, and collaborate on preclinical and clinical research. The groups are facilitated with CBTN by allowing for sharing of resources, clinical data, biospecimens, preclinical models and genomic and proteomic data that can be used to develop new data-driven clinical trials.
If you would like to participate or learn more about the CBTN/PNOC working groups, please email Ryan Velasco, Research Program Coordinator, (velascor@chop.edu).
CBTN/PNOC WORKING GROUP LEADS
MEDULLOBLASTOMA LEADS
- Robert Wechsler-Reya, PhD; Sanford Burnham Prebys
- Tabitha Cooney, MD; UCSF Benioff Children’s Hospital
CRANIOPHARYNGIOMA LEADS
- Cassie Kline, MD; Children’s Hospital of Philadelphia
- Fatema Malbari, MD; Texas Children’s Hospital
LOW GRADE GLIOMA LEADS
- Angela Waanders, MD, MPH; Ann & Robert H. Lurie Children’s Hospital of Chicago
- Daphne Adele Haas-Kogan, MD; Dana-Farber Cancer Institute
- Pratiti Bandopadhayay, MBBS, PhD; Dana-Farber Cancer Institute
- Joanna Phillips, MD, PhD; UCSF Helen Diller Family Comprehensive Cancer Center
AT/RT LEADS
- Ashley Margol, MD, MS; Children’s Hospital Los Angeles
- Annie Huang, MD, PhD, FRCP(C); SickKids
- Eric Raabe, MD, PhD; Johns Hopkins School of Medicine
EPENDYMOMA LEADS
- Eugene Hwang, MD; Children’s National Hospital
- Mariella Filbin, MD, PhD; Dana-Farber Cancer Institute
- Steven Mack, PhD; Texas Children’s Hospital
- Derek Hanson, MD; Hackensack Meridian Health
DIFFUSE MIDLINE GLIOMA LEADS
- Sabine Mueller, MD, PhD; Children’s Hospital Zurich
- Javad Nazarian, PhD; Children’s Hospital Zurich
IMAGING WORKING GROUP LEADS
- Josh Rubin, MD, PhD; Washington University School of Medicine, St. Louis
- Javier Villaneuva-Meyer, MD; University of California San Francisco
- S. Ali Nabavizadeh, MD; University of Pennsylvania
OPEN PBTA
Developed by the CBTN in collaboration with the Pacific Pediatric Neuro-Oncology Consortium (PNOC), the Pediatric Brain Tumor Atlas (PBTA) is a global effort to accelerate discoveries for therapeutic intervention for children diagnosed with brain tumors through the non-embargoed, pre-publication release of one of the largest genomic datasets for pediatric brain tumors to date. This foundational dataset was released on the NIH Common Fund-supported Gabriella Miller Kids First Data Resource Portal and CAVATICA workspace.
In FY20, CBTN researchers at Children’s Hospital of Philadelphia (CHOP) and the Childhood Cancer Data Lab (CCDL) at Alex’s Lemonade Stand Foundation set out to launch the OpenPBTA Project, a first-in-kind collaborative, crowd-sourcing analytic global initiative. This new open science project seeks to harness the collective expertise of the biomedical research and data informatics community to comprehensively describe the PBTA and accelerate scientific discoveries that will lead to clinical impact for childhood cancer patents.
The OpenPBTA Project is organized on GitHub, which allows data scientists to work together. Bioinformaticians and data scientists anywhere on earth are invited to join the project at the OpenPBTA GitHub Analysis Repository. This crowd-sourced, open-analysis effort is contributing to a manuscript that will provide a detailed, molecular picture of the more than 30 pediatric brain tumor types represented within the PBTA. The completed manuscript will help laboratory scientists and clinicians to more clearly identify, describe, and chart how cancerous brain cells mutate and propagate separate from normal tissue cells. The OpenPBTA manuscript will continue to evolve and expand, with updates available in real-time and on an on-going basis. This will allow for continuous team science efforts and provide an expanding resource to empower additional scientific discoveries. To learn more about the Open PBTA initiative, visit cbtn.org or ccdatalab.org/openpbta.
GIFT FROM A CHILD
Gift from a Child (GFAC), supported by the Swifty Foundation, ensures patients and families can donate samples of brain tumor tissue at the end of life. The gift of postmortem tissue donation benefits other children diagnosed with a pediatric brain tumor and can provide families with hope and consolation during a time of loss.
GFAC Centers of Excellence, including the newest site at Weill Cornell Medicine, work to collect, process and track donations to the CBTN. This initiative allows each donated sample to provide the greatest possible impact on children’s brain tumor research. The collection of these samples is key to further understand the biological and molecular changes that occur within brain tumors, especially in tumors that were not surgically possible to biopsy or resect due to location. In addition to tumor tissue, this effort allows the collection of normal brain tissue samples to use for comparison.
To learn more about Gift from the Child initiative, visit cbtn.org or giftfromachild.org.
NF2 BIOSOLUTIONS
To support expanded cross-disease research, CBTN partnered with NF2 Biosolutions, a nonprofit organization dedicated to finding an effective treatment or cure for Neurofibromatosis 2 (NF2). NF2 is a disease characterized by tumors on the spinal and cranial nerves, with over fifty percent of the cases inherited. The afflicted often suffer hearing and vision loss.
By leveraging the CBTN’s infrastructure and resources, NF2 Biosolutions will refer patients to CBTN Clinical Research Coordinators, who will consent and coordinate the collection of samples and data from previous and upcoming surgeries. Any data generated will be available for research and also allow cross-disease analysis to expand potential findings.
To learn more, visit nf2biosolutions.org
The expansion of CAVATICA has already enhanced international collaboration by providing easier and faster approaches to sharing data and analyzing rare childhood cancer data.
Since we know so little about many pediatric cancer subtypes, it is essential that we share data globally. We may be able to better understand how to treat an Australian patient’s tumour by comparing it to larger numbers of American patients.
The innovative technology of CAVATICA enables researchers from Australia and the United States to seamlessly share data and novel analysis methods with ease, driving improved outcomes and novel research.
CAVATICA AUSTRALIA
Beginning in February of 2020, The Children’s Brain Tumor Network joined an international alliance including the Australian Research Data Commons (ARDC), Bioplatforms Australia, the Australian BioCommons, Children’s Cancer Institute in Australia, The Gabriella Miller Kids First Data Resource Center (Kids First DRC), and Seven Bridges Genomics to expand use of the CAVATICA data analysis platform into Australia, utilizing Amazon Web Services in Sydney and piloting new capabilities to support Australian initiatives such as Zero Childhood Cancer (Zero).
These CBTN partners aim to build a computational infrastructure that will harmonize data from Zero, with the genomic datasets of CBTN and the Kids First DRC. The effort will help to improve researchers’ understanding of rare pediatric brain cancer subtypes.
In late 2018, the Kids First DRC launched an open-source research portal for pediatric cancers and structural birth defects which includes DNA and RNA samples from CBTN. The Kids First DRC, which is supported through the National Institutes of Health (NIH) Common Fund’s Gabriella Miller Kids First Pediatric Research Program, also uses the CAVATICA platform for storing, sharing, and analyzing large volumes of pediatric genomic data.
OLIGONATION
Oligodendroglioma is rare, occurring in less than one percent of pediatric brain tumor patients under 14 years of age, although slightly more often in teens and young adults. These and other factors present challenges to identify clinical trials or promising research advancements for young people who develop an Oligodendroglioma. And because Oligodendroglioma tends to be a slow growing cancer, the lack of short term ‘endpoints’ for Oligo trials makes it more difficult to recruit support from researchers, drug companies, and government agencies such as the Federal Drug Administration (FDA).
Without sufficient data to develop hypotheses or the preclinical tools to test them, progress is difficult. To address these needs, OligoNation was founded in 2014 and has since raised over $1.3 million for research. With an initial emphasis on translational research, OligoNation is now focusing efforts on biobanking and facilitating preclinical research (cell-line and PDX model development) that will lead to more clinical trials for Oligodendroglioma.
As CBTN and OligoNation align on many of the same goals, a new partnership was formed during FY20 to establish a pipeline for gathering Oligodendroglioma tissue samples and providing data to the research community. A collated sample listing from across CBTN, the University of Pennsylvania and OligoNation have undergone whole-genome and RNA sequencing. Once analysis of the 122 DNA and 62 RNA Oligo cohorts is completed, the findings will be made available to benefit the research and patient community.
Scientific Community Resources
USER SUPPORT OFFICE HOURS
To provide technical support and resources for the CBTN research community, ‘user support office hours’ were instituted during FY20. These open information sessions allow researchers to ask questions or view tutorials from experts on the CBTN Operations Team. Topics range from how to navigate CBTN’s analysis platforms, to querying data, to discovering or creating pipelines and workflows
Researchers and clinicians seeking to learn more about the CBTN’s available pediatric brain tumor data are invited to join each monthly session. During these sessions, tutorials and support topics for using platforms including CAVATICA, PedcBioPortal and the Gabriella Miller Kids First Data Research Portal are available upon request.
INTERESTED IN JOINING OFFICE HOURS?
Contact a member of the CBTN operations team for meeting information, to submit questions, or suggest discussion topics.
Email us at reseach@cbtn.org
EXPANDED RESOURCE REQUESTS
After receiving feedback from researchers about the CBTN’s specimen request process, CBTN has phased out its quarterly request periods, allowing researchers to submit specimen requests at any time. This update was made possible through the formation of two review groups within the CBTN Scientific Committee. Members of the CBTN Scientific Committee review and provide feedback and/or approval for all submitted project requests. The CBTN also accepts requests for cell lines and data projects year-round, which are available to researchers anywhere on earth.
NEED TO REQUEST SPECIMENS OR DATA?
For information and instructions on submitting a request to CBTN biosamples, cell lines and data, visit cbtn.org/research-resources
Advancing Our Mission

CBTN Executive Board FY21 Objectives
DRIVING INNOVATIVE, PIONEERING, OPEN SCIENCE
Objective 1
Strategically growing the number of CBTN members in the U.S. and internationally will drive the availability, utilization and increased volume of pediatric brain tumor resources, research, and increase the network of collaborative innovative experts overcoming the inter-related barriers to advancing pediatric brain tumor research.
Objective 2
Increase the number of U.S. and international research projects, and discoveries supported by CBTN aligned to the open science model for release without embargo of data, biospecimens and preclinical models throughout the research community.
Objective 3
Increase the number of U.S. and international precision medicine clinical trials and therapeutic treatment decision mechanisms supported by CBTN’s pediatric and AYA data to allow clinical trials to be designed and supported by pediatric and AYA brain tumor data.
Objective 4
Improve equity of global access to healthcare and to clinical trials by developing CBTN led research looking at health disparities in pediatric brain tumor patients.
Objective 5
Increase and drive patient and foundation collaborations to develop partnered research projects, clinical trials, collaborative opportunities aligned with CBTN’s vision and mission to improve treatments, outcomes and quality of life.
DEVELOPING AND SUSTAINING ESSENTIAL STRATEGIC PARTNERSHIPS
Objective 1
Increase and drive governmental pediatric brain tumor research in the U.S. and internationally for increased resources, collaboration and expertise in pediatric brain and AYA brain tumor.
Objective 2
Increase and drive commercial partnerships to drive the utilization of first class innovative solutions and technology for pediatric brain tumor research.
Objective 3
Establish pharmaceutical partnerships for the development of pediatric brain tumor clinical trials and therapies supported by CBTN.
DRIVING INNOVATIVE, PIONEERING, OPEN SCIENCE
Objective 1
Strategically growing the number of CBTN members in the U.S. and internationally will drive the availability, utilization and increased volume of pediatric brain tumor resources, research, and increase the network of collaborative innovative experts overcoming the inter-related barriers to advancing pediatric brain tumor research.
Moving Our Mission Forward
NEW CBTN WEBSITE
Growth from CBTTC to CBTN has continued into FY21 with the launch of our redesigned website (cbtn.org) in October 2020. With a greater focus on the CBTN’s scientific discovery projects, the new CBTN website provides a opportunities for visitors to explore, learn, and connect with CBTN investigators, foundations, and research partners.
CBTN website visitors can explore the biospecimen and data project pages while learning more about the research teams, institutions, and foundations involved with each project. Users can also view project goals, funding needs and each project’s impact to the CBTN. The redesigned member institution pages allow visitors to learn more about the investigators, clinicians, research coordinators, and other CBTN research team members at each CBTN member institution. Each section of the website is linked to other relavant informational cards including publications, presentations and news items.
Additional website features highlight CBTN’s research findings, including a ‘Publications’ section, an ‘About Us’ section which shares how CBTN empowers discovery through data-driven research, foundation partner pages which highlight the critical role of the patient communities in the CBTN mission, a newly-designed donation platform, and much more.
CBTN/CPTAC PROTEOMICS PAPER
CBTN and the Clinical Proteomic Tumor Analysis Consortium (CPTAC) collected and analyzed genetic, genomic, and proteomic data from multiple types of brain tumors in children. The research team consists of collaborators from the Icahn School of Medicine at Mount Sinai, the National Cancer Institute, Fred Hutchinson Cancer Research Center, Children’s National Hospital and Children’s Hospital of Philadelphia.
This paper is the first large-scale multicenter study focused on pediatric brain tumors, available online in Cell on Nov. 25, 2020. A comprehensive “proteogenomic” analysis of the proteins, genes, and RNA transcription involved in pediatric brain tumors has yielded a more complete understanding of these tumors, which are the leading cause of cancer-related death in children. The results could help physicians more accurately identify different types of tumors and methods for treating them.
This study is the first comprehensive survey of genomics (aims to characterize DNA sequence alterations in samples), transcriptomics (aims to quantify copies of RNAs), global proteomics (aims to identify and quantify proteins), and phosphoproteomics (quantifies active proteins) across a large cohort of 218 tumor samples representing seven distinct types of brain tumors.
By characterizing biological themes that are shared among these different types of tumors, the study revealed new insights which suggest that current treatments being used for specific tumor types could be applied to others that share the same proteomic features. Through leveraging the rich clinical outcome data of this cohort, the research team also identified new prognostic biomarkers for a type of tumor known as high-grade glioma (HGG). When HGG tumors have a genetic mutation known as a H3K27M mutation, they tend to be very aggressive and the patients have relatively short survival time. However, in those without the mutation, this study suggests that the abundance of proteins named IDH1 and IDH2 in the tumor tissues could help to identify which tumors with the non-mutated H3K27M gene may be less aggressive.
The data analysis also showed key biological differences in samples from primary and recurrent tumors from the same patients, indicating the need for independent assessment and therapeutic decisions for these tumors.
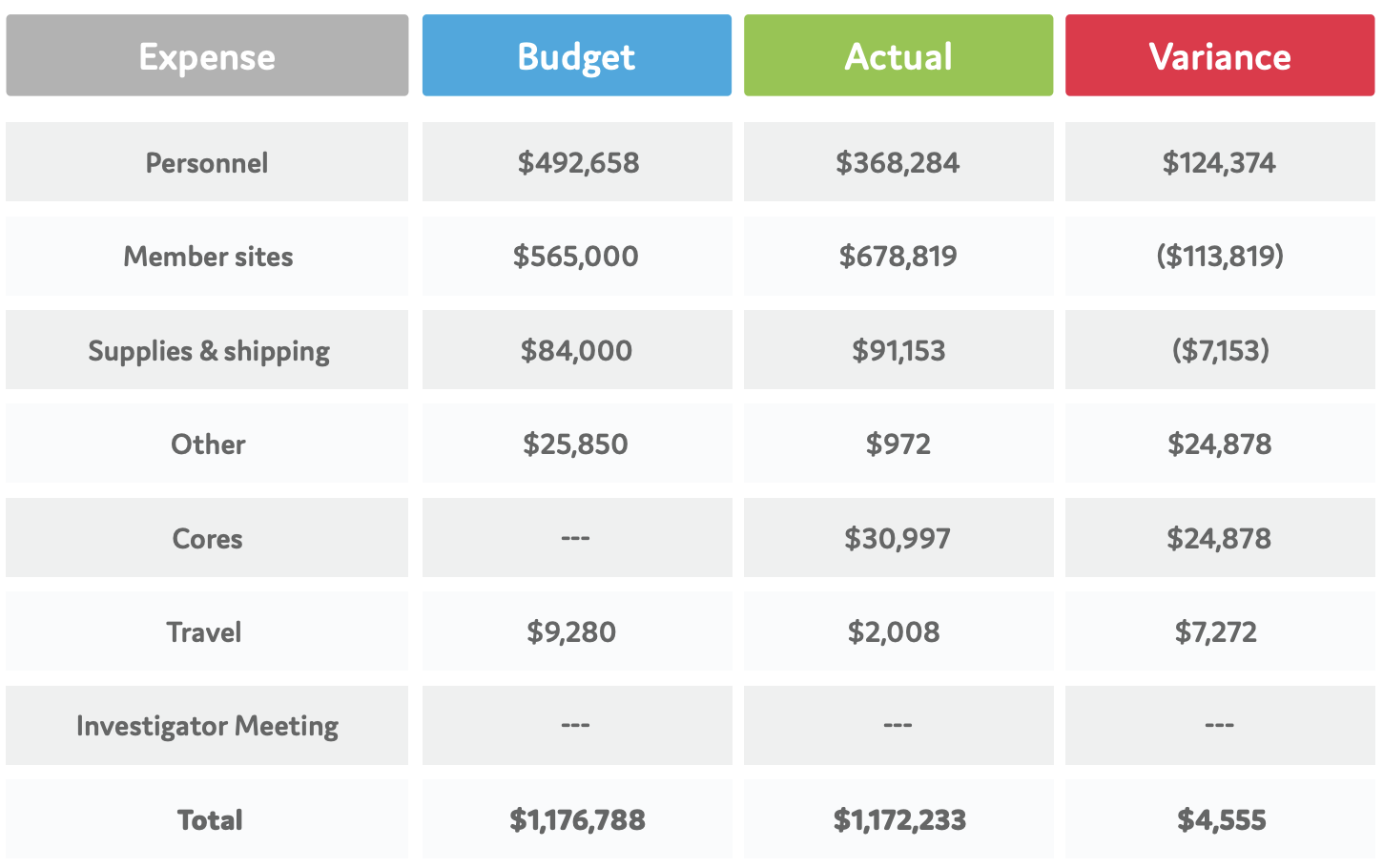
Financial Summary
FY19-20 CBTN FINANCIAL SUMMARY
The CBTN is truly grateful to have been supported by Children’s Hospital of Philadelphia, including the Division of Neurosurgery and the Center for Data Driven Discovery in Biomedicine (D3b), as well as the generosity of individuals, families and foundations since its inception. These philanthropic efforts highlight the network of partners who are dedicated to the CBTN’s mission and vision.
Note: Other resources provided by Children’s Hospital of Philadelphia, not reflected in these numbers, include use of the Hospital’s state-of-the-art biorepository, bioinformatics platforms developed through other grants and mechanisms, additional staff support from the more than 60 members of the D³b Center, and gift processing and donor relations support from CHOP Foundation.