The landscape of genomic alterations across childhood cancers
Abstract
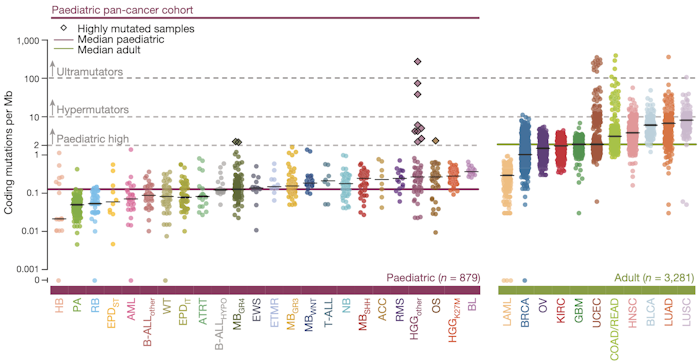
Pan-cancer analyses that examine commonalities and differences among various cancer types have emerged as a powerful way to obtain novel insights into cancer biology. Here we present a comprehensive analysis of genetic alterations in a pan-cancer cohort including 961 tumours from children, adolescents, and young adults, comprising 24 distinct molecular types of cancer. Using a standardized workflow, we identified marked differences in terms of mutation frequency and significantly mutated genes in comparison to previously analysed adult cancers. Genetic alterations in 149 putative cancer driver genes separate the tumours into two classes: small mutation and structural/copy-number variant (correlating with germline variants). Structural variants, hyperdiploidy, and chromothripsis are linked to TP53 mutation status and mutational signatures. Our data suggest that 7–8% of the children in this cohort carry an unambiguous predisposing germline variant and that nearly 50% of paediatric neoplasms harbour a potentially druggable event, which is highly relevant for the design of future clinical trials.