Frank M. Balis
Philadelphia, PA USA
Children’s Hospital of Philadelphia

About
Director, Cancer Clinical Research Institutional Principal Investigator, Children's Oncology Group; Attending Physician The Louis and Amelia Canuso Family Endowed Chair for Clinical Research in Oncology;Professor of Pediatrics, Perelman School of Medicine
Children's Hospital of Philadelphia
As CHOP’s Institutional Principal Investigator for the Children’s Oncology Group (COG), I oversee the Cancer Center’s substantial contributions to the national clinical research efforts of the COG. We are striving to be one of the premier pediatric oncology clinical research program in the COG.
My clinical research focuses on characterizing the clinical pharmacology of anticancer drugs in children in order to optimize their effectiveness and minimize their side effects, and on the development of new drugs and treatment strategies for childhood cancers. The dose and administration schedule of a drug, its effectiveness against childhood cancers, and the types and severity of side effects that can occur in children cannot be predicted from the experience with the drug in adults. As a result, we test new drugs in children separately by evaluating side effects at varying dose levels, by measuring the response of the cancer, and by studying their clinical pharmacology by measuring the amount of drug in the body and its rate of elimination.
Anticancer drug development has been revolutionized by our increasing knowledge of the genetic and molecular defects in the various types of cancer that cause a cell to become cancerous. New classes of anticancer drugs specifically target these defects; as a result, they can more selectively block the growth of cancer cells. These new anticancer drugs hold the promise of being more effective and less toxic. This includes biological agents, which are playing an increasingly important role in the treatment of childhood cancers, such as immunotherapy. The applicability of these new classes of drugs to childhood cancers and the need to develop new approaches to study these drugs in children with cancer are new challenges that our drug development program has the experience and expertise to address.
Biomarkers are essential tools in clinical practice and clinical research. Tumor biomarkers are useful for screening or early detection of cancers, for diagnosis and determining prognosis, and for predicting response to treatment, monitoring response and detecting relapse or tumor progression. Research applications of tumor biomarkers parallel their clinical uses. We have identified GD2 as a biomarker for high-risk neuroblastoma. GD2 may be useful as a diagnostic and prognostic tumor biomarker, and we are studying it in a prospective national clinical trial. We are also studying biomarkers of normal tissue or organ damage to more sensitively detect and measure drug toxicity.

Children’s Hospital of Philadelphia
research
Interests
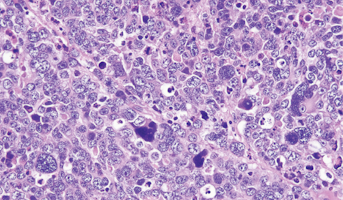
Medulloblastoma
Medulloblastomas comprises the vast majority of pediatric embryonal tumors and by definition arise in the posterior fossa, where they constitute approximately 40% of all posterior fossa tumors. Other forms of embryonal tumors each make up 2% or less of all childhood brain tumors.The clinical feature
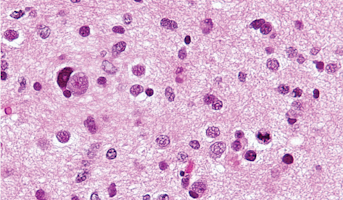
High-Grade Glioma
High-grade Gliomas (HGG) or astrocytomas in children nearly always result in a dismal prognosis. Although novel therapeutic approaches are currently in development, preclinical testing has been limited, due to a lack of pediatric-specific HGG preclinical models. These models are needed to help test

